November 3, 2020 to November 6, 2020
ONLINE
REGISTRATION IS CLOSED.
Registration occurs on a first-come, first-served basis. The deadline for registration is one week before the first day of the course. If you are unable to register before the deadline, please email: registrar@faes.org or call 301-496-7977 for space availability.
NIH Fellows or NIH community members being sponsored by their lab and awaiting payment authorization can tentatively hold a seat using the “Reserve A Seat” option. FAES must receive payment within 7 business days after reserving a seat or 3 business days before the start of the workshop, which ever comes first. If payment is not received in this time frame, your reservation will be canceled.
Background
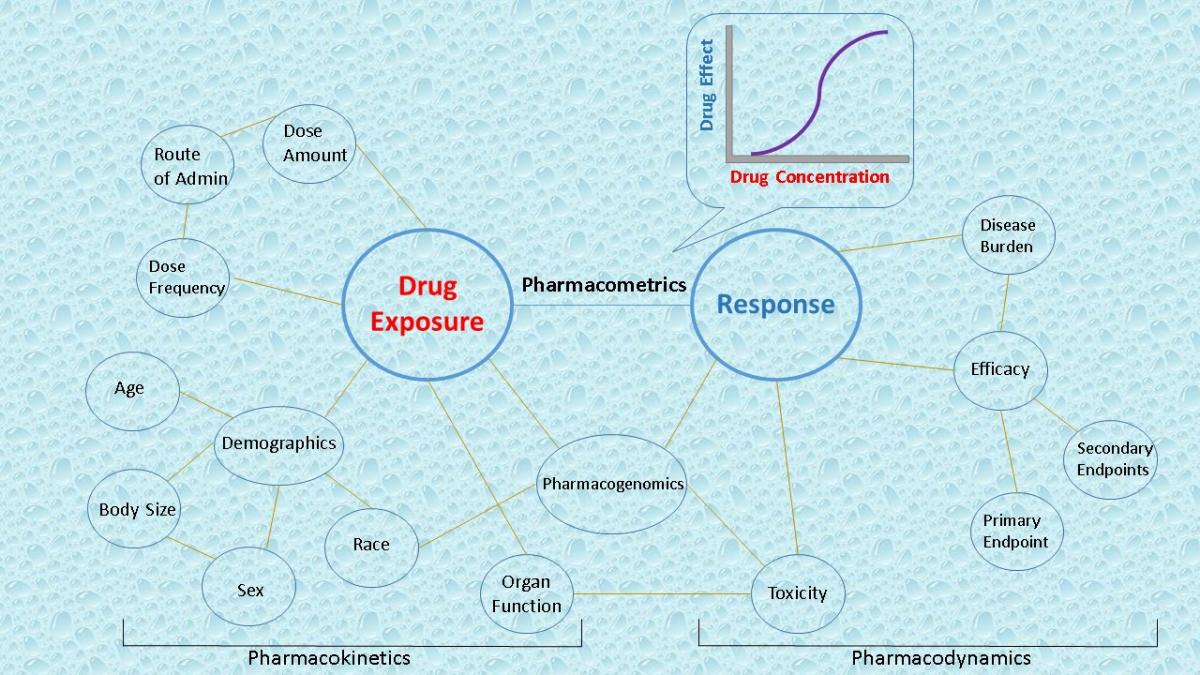
In order for a drug to get approved by the FDA for market in the USA, the sponsor must ultimately demonstrate the drug has: 1) a predictable exposure profile with dose, 2) a good safety profile, and 3) is effective at safe doses. Therefore, the pharmacology of a drug is essentially being reviewed by the FDA. The ability of scientists to analyze drug exposure/response relationships is crucial to understanding what exposure amount will elicit the safest, most effective response, and ultimately what dose amount and frequency will produce the optimal exposure amount. Additionally, the ability to identify sub-populations that may produce differing exposure or response levels is key to providing as many subjects as possible a safe and effective dose. This quantitative exposure/response analyses, often referred to pharmacometrics, is key to making go/no go decisions both during clinical trials by investigators, and the FDA during the subsequent review period.
Simultaneous access to two screens is highly recommended for best learning experience. Examples include two laptops, one computer with two screens, one laptop and one tablet, etc.
Objectives
Participants will learn basic pharmacology theory with introductory statistics using a popular open source software program (R Studio) that is capable of conducting pharmacokinetic (PK) exposure and pharmacodynamic (PD) response analyses from example clinical trial data. Ultimately, the framework of analyzing exposure/response relationships will be demonstrated in order to make go/no go decisions.
Highlights
1. Introduction to a broad range of pharmacology topics
2. Introduction to a basic level of statistical analyses for clinical trial data
3. Introduction to the basics of computer coding necessary for R Studio
4. Framework of analyses laid for post-course continuing education
Who should attend
1. Clinicians and researchers interested in learning how to utilize freely available software to explore, visualize and understand drug exposure/response relationships, where responses include any clinical endpoint collected on a trial.
2. Clinicians and researchers interested in understanding and predicting the effect of different doses on drug exposure, and effect of exposure on a variety of clinically-relevant response endpoints (biomarkers).
3. Medical, pharmacy, dental, nursing, and lab-based graduate school students interesting in obtaining a deeper understanding of pharmacokinetics, exposure/response analyses, and a broad understanding of clinical drug development and the impact of pharmacometrics on decisions.
Hands-on Skills/Tools Taught
1. Noncompartmental analysis of pharmacokinetic data using R Studio
2. Statistical hypothesis testing of clinical trial data using R Studio
3. Exposure/response analyses using R Studio
Workshops generally run from 9:00am - 5:00pm.
Simultaneous access to two screens is highly recommended for best learning experience. Examples include one computer with two screens, two computers, one laptop and one tablet, etc.
About the Instructor
Dr. Peer is a pharmacologist in the Clinical Pharmacology Program at the National Cancer Institute. He received his graduate training at the University of Maryland training program where he obtained a Masters in Pharmacometrics as well as a PhD in pharmacology and pharmaceutical sciences at West Virginia University. Following his fellowship with the National Cancer Institute, he was retained as an Staff Scientist within the NCI.
General Training Rate
$1,199.00
Discounted Training Rates
$695.00 - NIH Trainees (Fellows, PostDocs, PostBacs working at the NIH Campus)
$895.00 - NIH Community (Working, Appointed, or Assigned to the NIH Campus)
$999.00 - Academia, US Government, US Military
Credit
Although no grades are given for courses, each participant will receive Continuing Education Units (CEUs) based on the number of contact hours. One CEU is equal to ten contact hours. Upon completion of the course each participant will receive a certificate, showing completion of the workshop and 2.8 CEUs.
Refund Policy
100% tuition refund for registrations cancelled 14 or more calendar days prior to the start of the workshop.
50% tuition refund for registrations cancelled between 4 to 13 calendar days prior to the start of the workshop.
No refund will be issued for registrations cancelled 3 calendar days or less prior to the start of the workshop.
Notification
All cancellations must be received in writing via email to Ms. Carline Coote at registration@faes.org.
Cancellations received after 4:00 pm (ET) on business days or received on non-business days are time marked for the following business day.
All refund payments will be processed by the start of the initial workshop.
Return to Workshops & Conferences Calendar